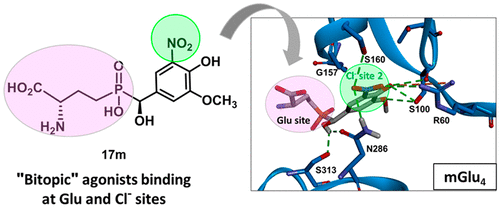
2,4‐Bis(fluoroalkyl)quinoline‐3‐carboxylates as tools for the development of potential agrochemical ingredients
F. Aribi, A. Panossian, J.-P. Vors, S. Pazenok, F. R. Leroux
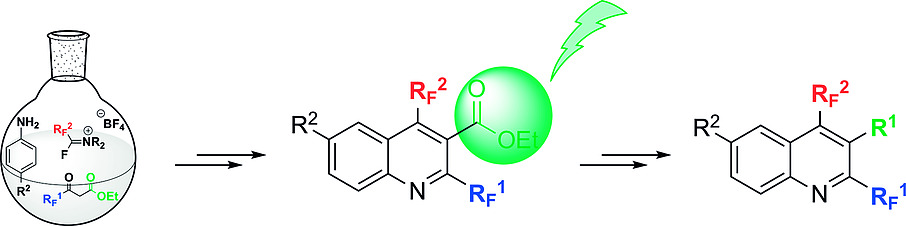
A Physico-chemical Investigation on Fluorine-Enriched Quinolines
F. Aribi, A. Panossian, D. Jacquemin, J.-P. Vors, S. Pazenok, F. R. Leroux, M. Elhabiri
J. Bortoluzzi, V. Jha, G. Levitre, M. J. Fer, J. Berreur, G. Masson, A. Panossian, F. R. Leroux
E. Schmitt, B. Commare, A. Panossian, J.-P. Vors, S. Pazenok, F. R. Leroux
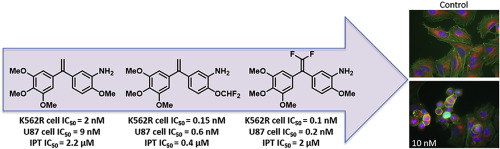
T. Nareta, J. Bignon, G. Bernadata, M. Benchekrouna, H. Levaique, C. Lenoir, J. Dubois, A. Pruvost, F. Saller, D. Borgel, B. Manoury, V. Leblaise, R. Darrigrand, S. Apcher, J.-D. Brion, E. Schmitt, F. R. Leroux, M. Alami, A. Hamze
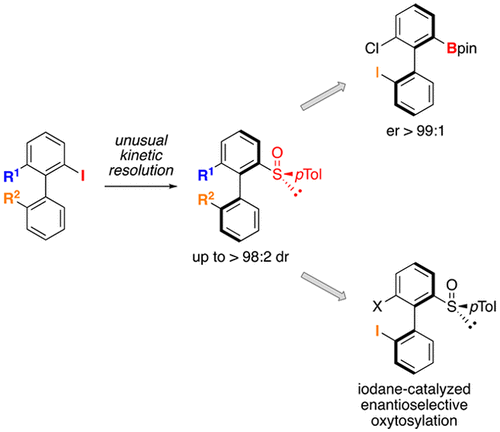
Access towards enantiopure α,α-difluoromethyl alcohols by means of sulfoxides as traceless chiral auxiliaries
C. Batisse, A. Panossian, G. Hanquet, F. Leroux
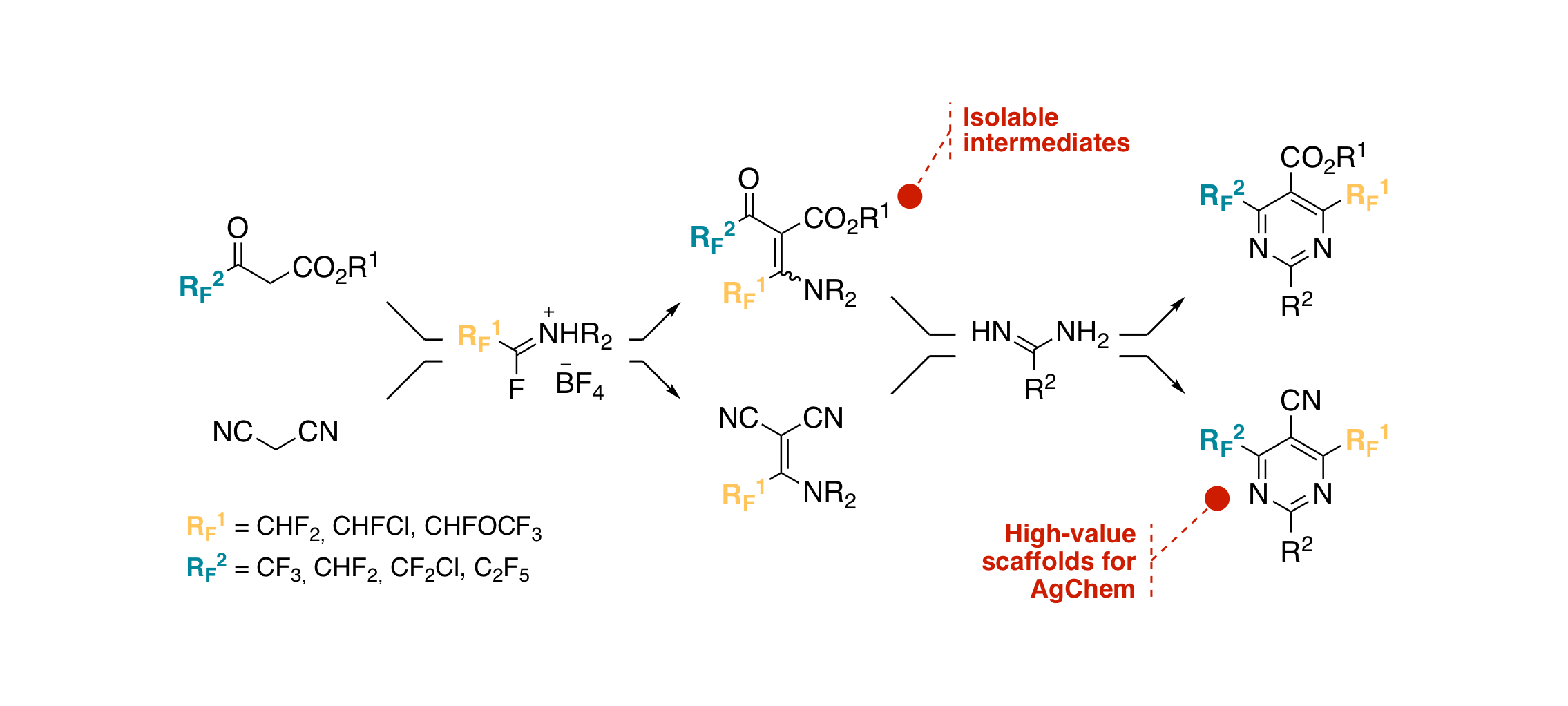
New synthetic access to 3-fluoroalkyl-5-pyrazolecarboxylates and carboxylic acids
A. Gómez Herrera, E. Schmitt, A. Panossian, J.-P. Vors, S. Pazenok, F. R. Leroux
