Want create site? Find Free WordPress Themes and plugins.
Fluoroalkyl Amino Reagents for the Introduction of the Fluoro(trifluoromethoxy)methyl Group onto Arenes and Heterocycles
E. Schmitt, S. Bouvet, B. Pégot, A. Panossian, J.-P. Vors, S. Pazenok, E. Magnier, F. R. Leroux
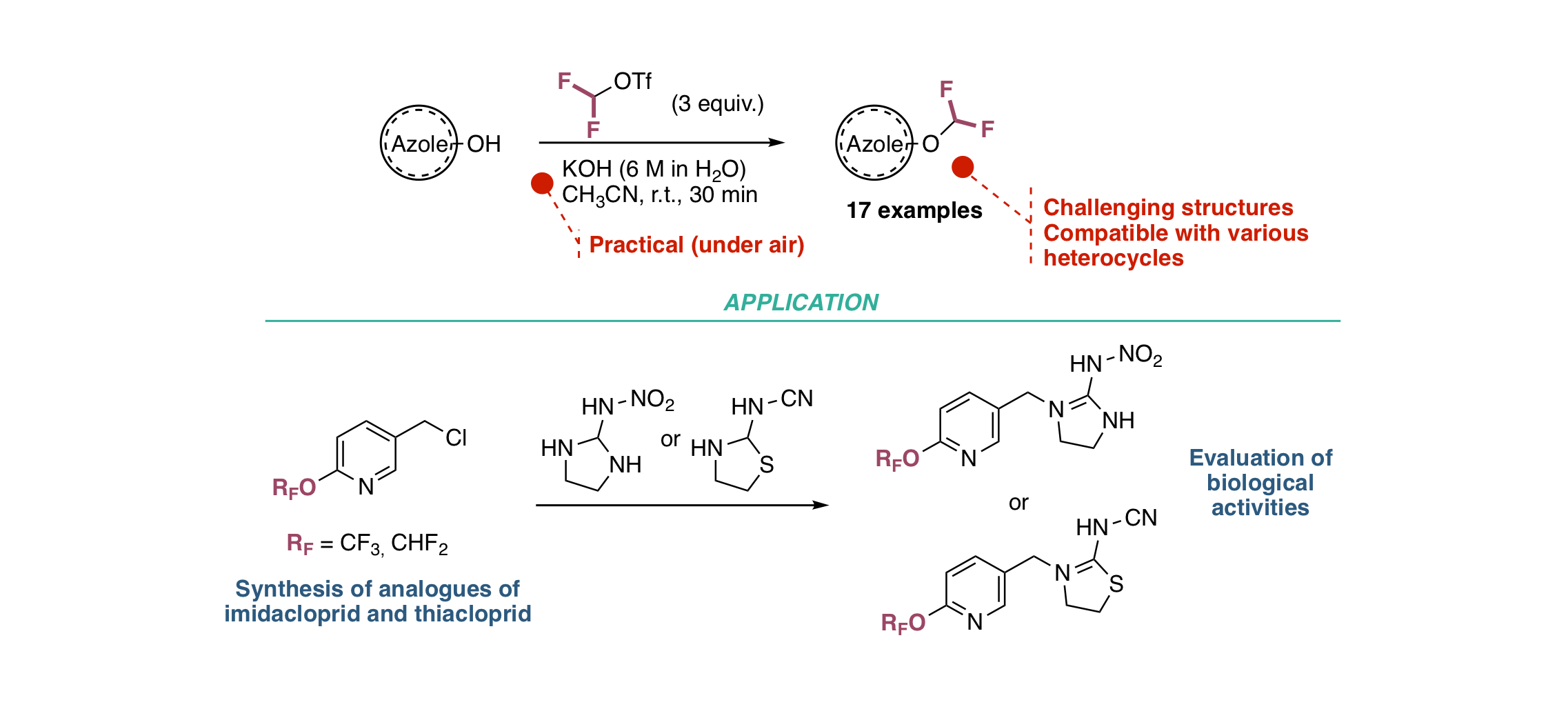
Tri- and difluoromethoxylated N-based heterocycles − Synthesis and insecticidal activity of novel F3CO- and F2HCO-analogues of Imidacloprid and Thiacloprid
G. Landelle, E. Schmitt, A. Panossian, J.-P. Vors, S. Pazenok, P. Jeschke, O. Gutbrod, F. R. Leroux
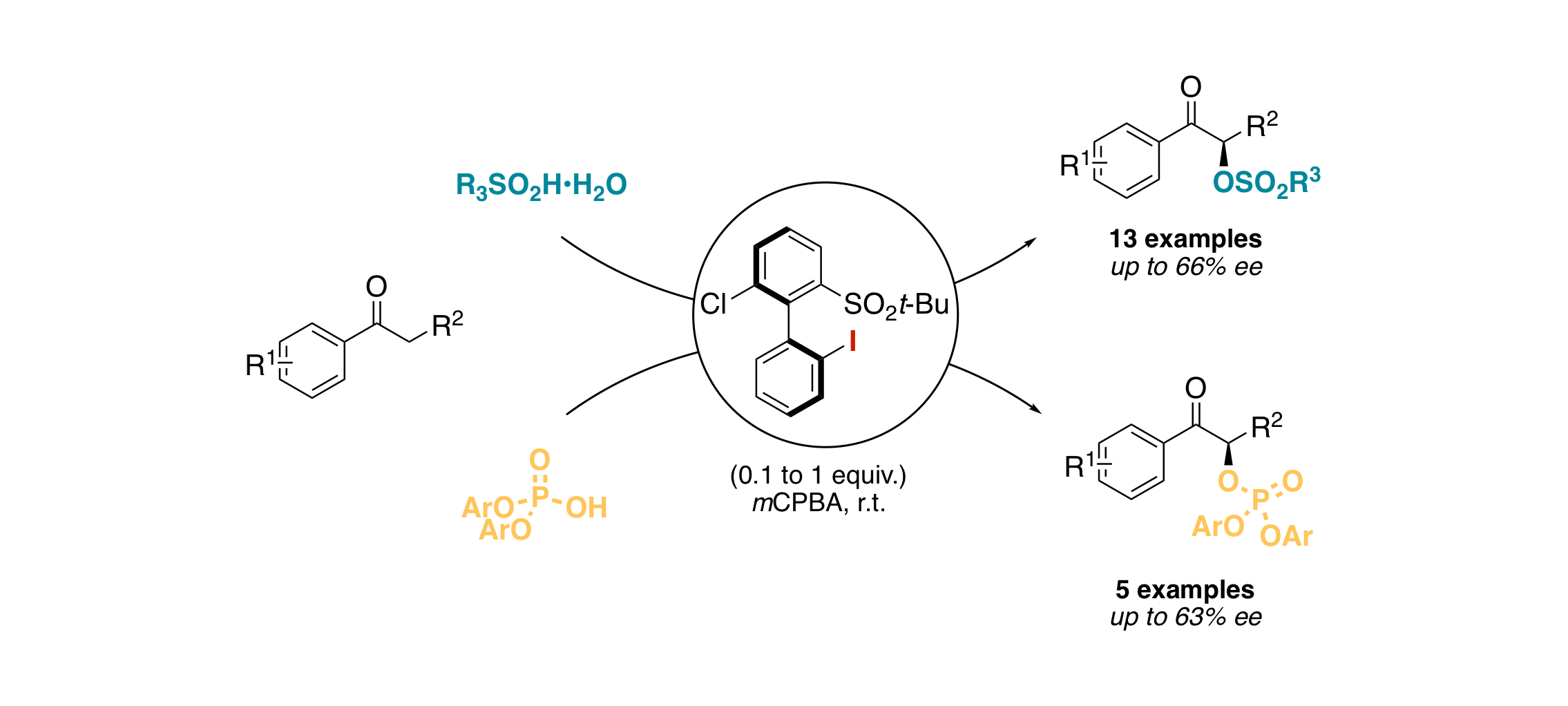
Asymmetric α-Sulfonyl- and α-Phosphoryl-Oxylation of Ketones by a Chiral Hypervalent Iodine(III)
G. Levitre, A. Dumoulin, P. Retailleau, A. Panossian, F. R. Leroux, G. Masson
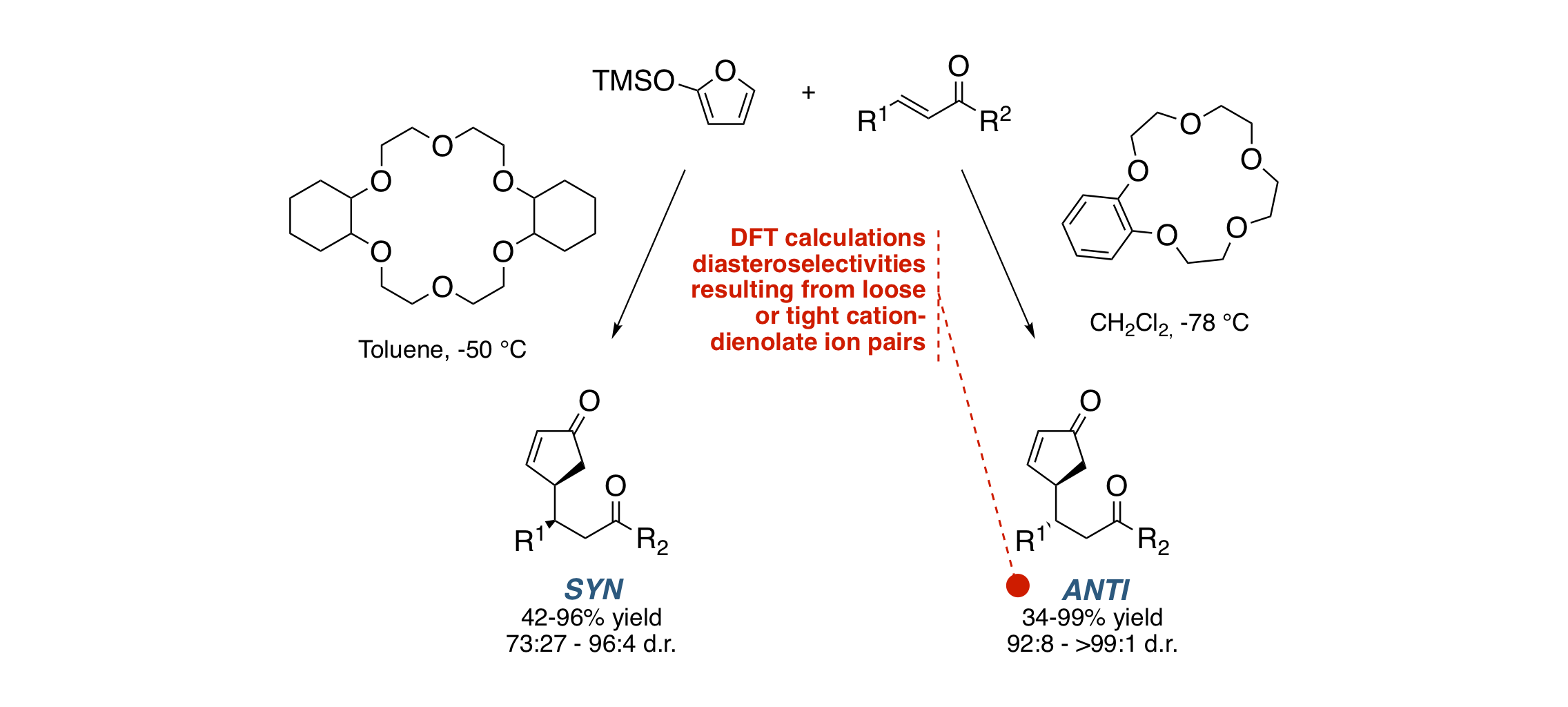
Switchable Diastereoselectivity in the Fluoride-Promoted Vinylogous Mukaiyama–Michael Reaction of 2-[(Trimethylsilyl)oxy]furan Catalyzed by Crown Ethers
G. Della Sala, M. Sicignano, R. Schettini, F. De Riccardis, L. Cavallo, Y. Minenkov, C. Batisse, G. Hanquet, F. R. Leroux, I. Izzo
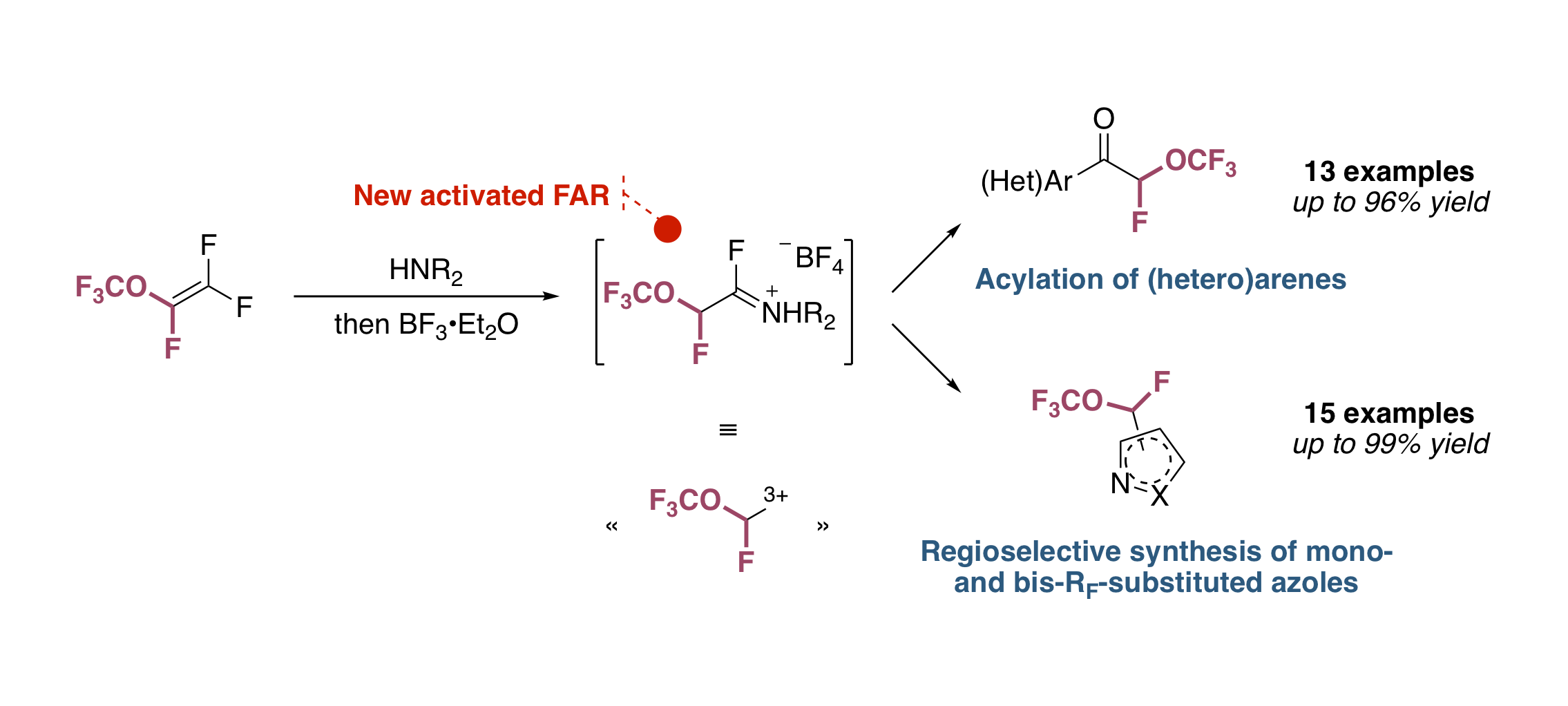
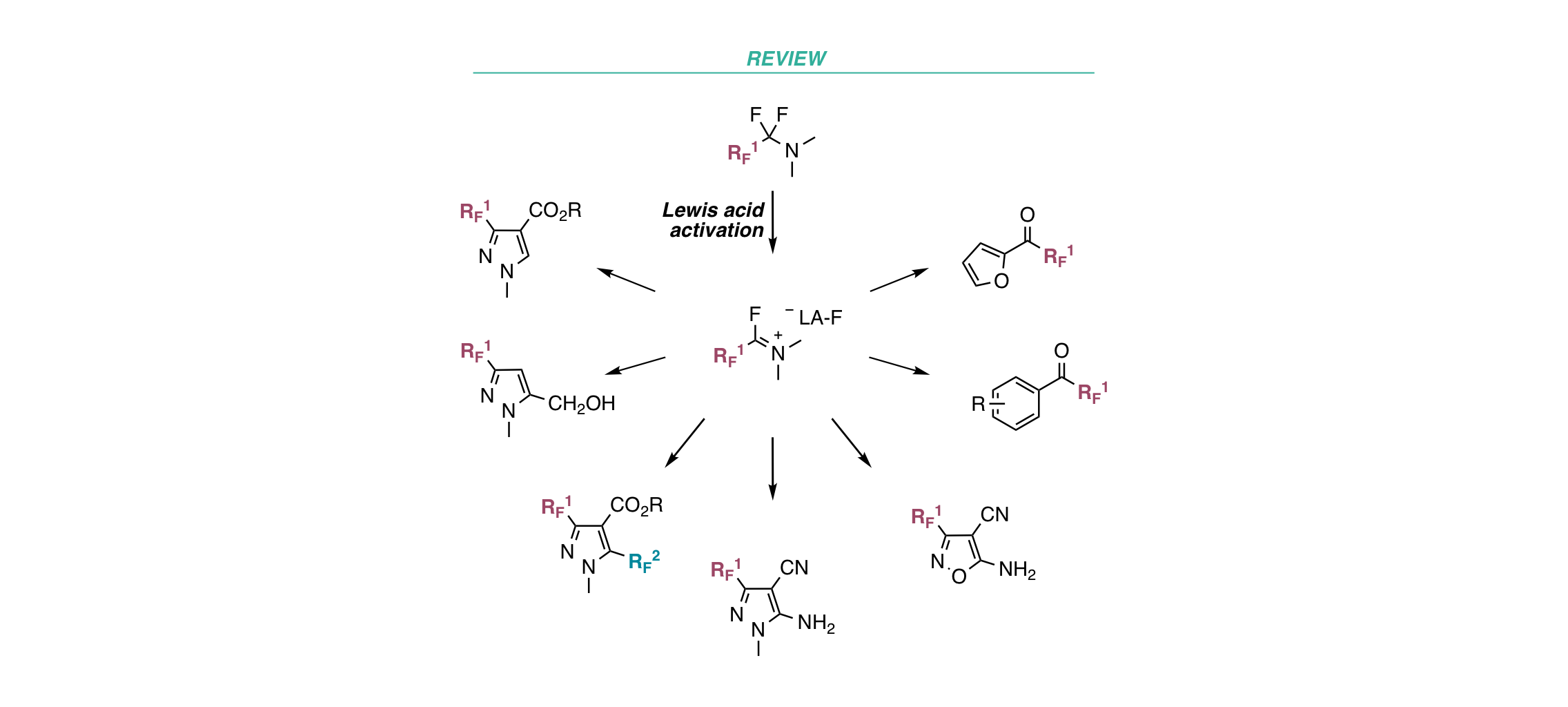
Fluoroalkyl Amino Reagents (FARs): A General Approach towards the Synthesis of Heterocyclic Compounds Bearing Emergent Fluorinated Substituents
B. Commare, E. Schmitt, F. Aribi, A. Panossian, J.-P. Vors, S. Pazenok, F. R. Leroux
Control of axial chirality in absence of transition metals based on arynes
F. R. Leroux, A. Panossian, D. Augros
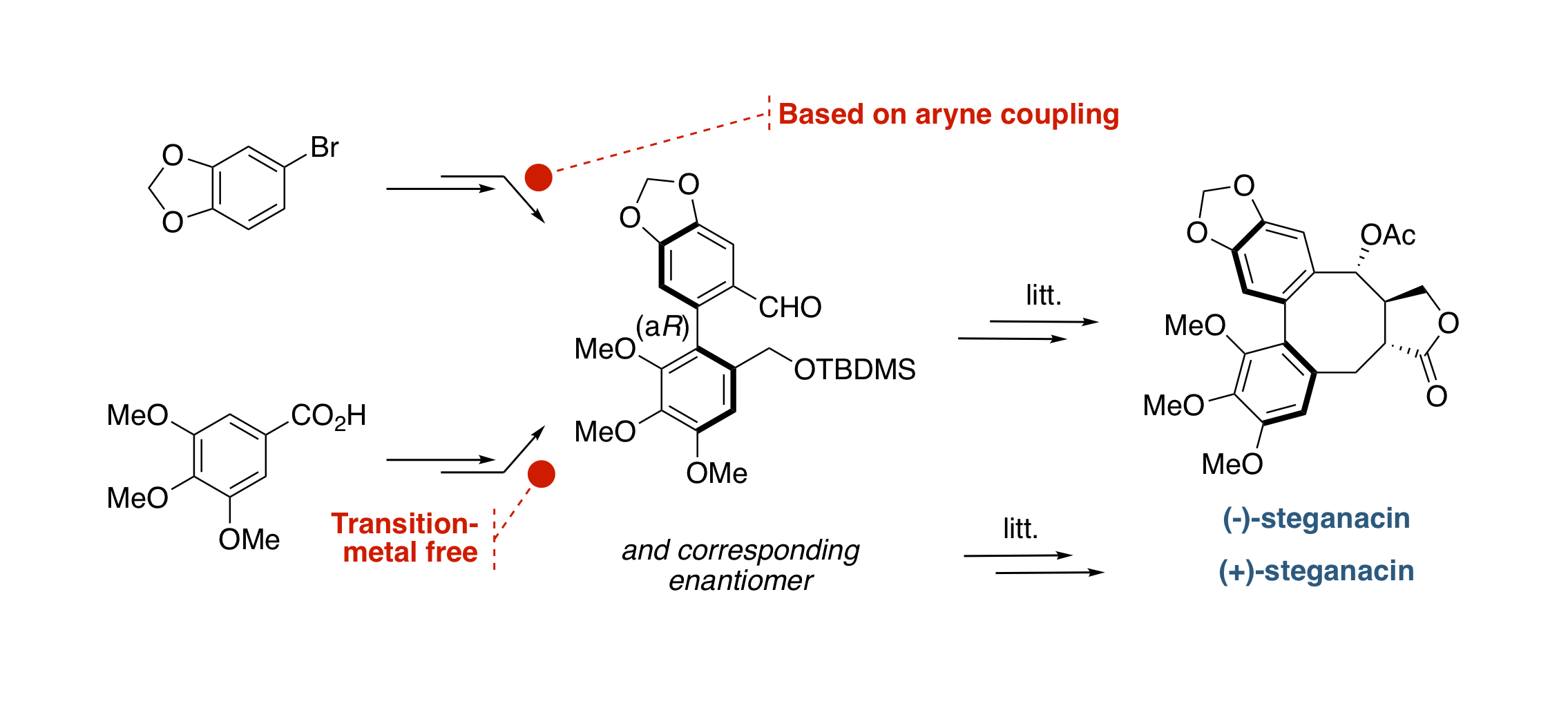
Transition-Metal-Free Synthesis of a Known Intermediate in the Formal Synthesis of (–)-Steganacin
D. Augros, B. Yalcouye, S. Choppin, M. Chessé, A. Panossian, F. R. Leroux
Did you find apk for android? You can find new Free Android Games and apps.
