P. Berdagué, J.-E. Herbert-Pucheta, V. Jha, A. Panossian, F. R. Leroux, P. Lesot
In Situ Generated Fluorinated Iminium Salts for Difluoromethylation and Difluoroacetylation
E. Schmitt, B. Rugeri, A. Panossian, J.-P. Vors, S. Pazenok, F. R. Leroux
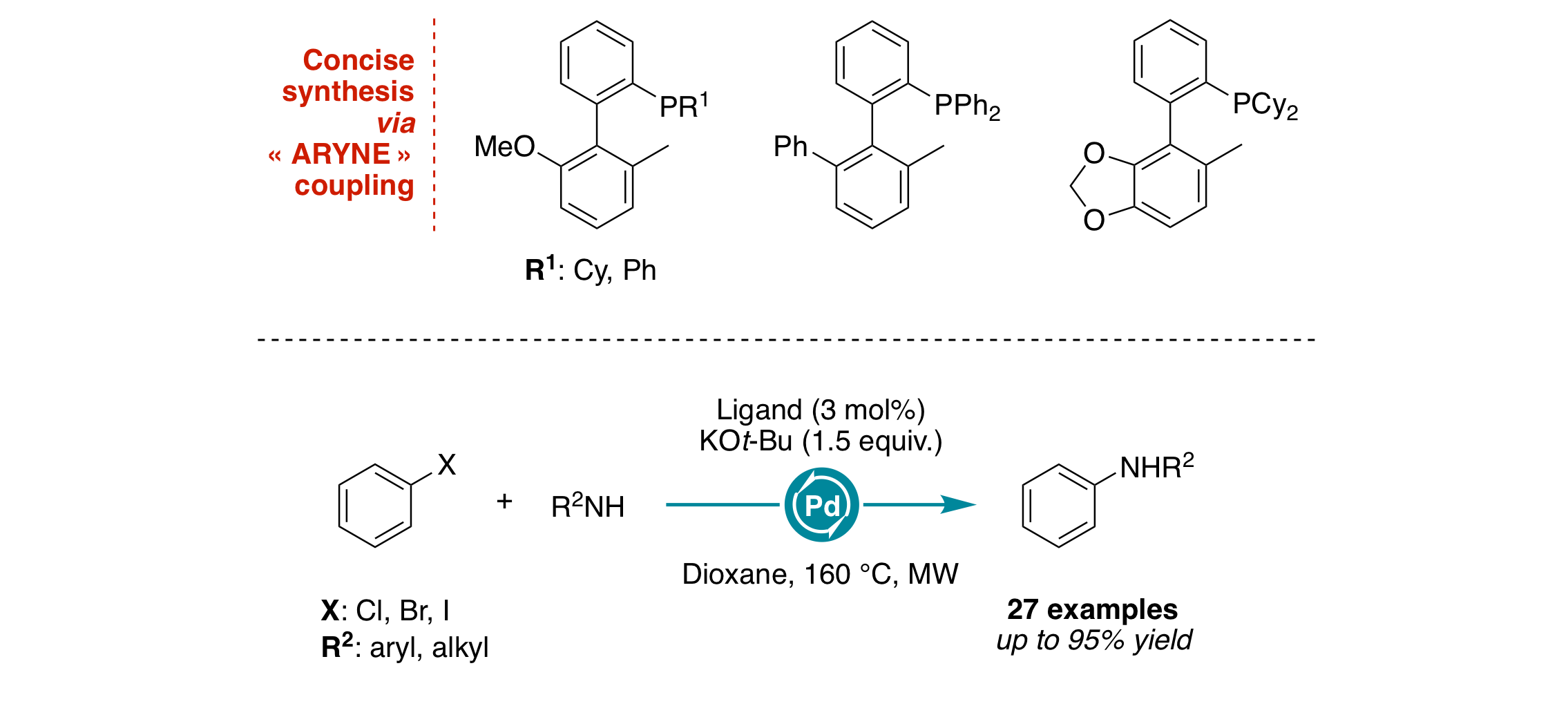
Modular Synthesis of Biaryl‐Substituted Phosphine Ligands: Application in Microwave‐Assisted Palladium‐Catalyzed C–N Cross‐Coupling Reactions
C. Singh, J. Rathod, V. Jha, A. Panossian, P. Kumar, F. R. Leroux
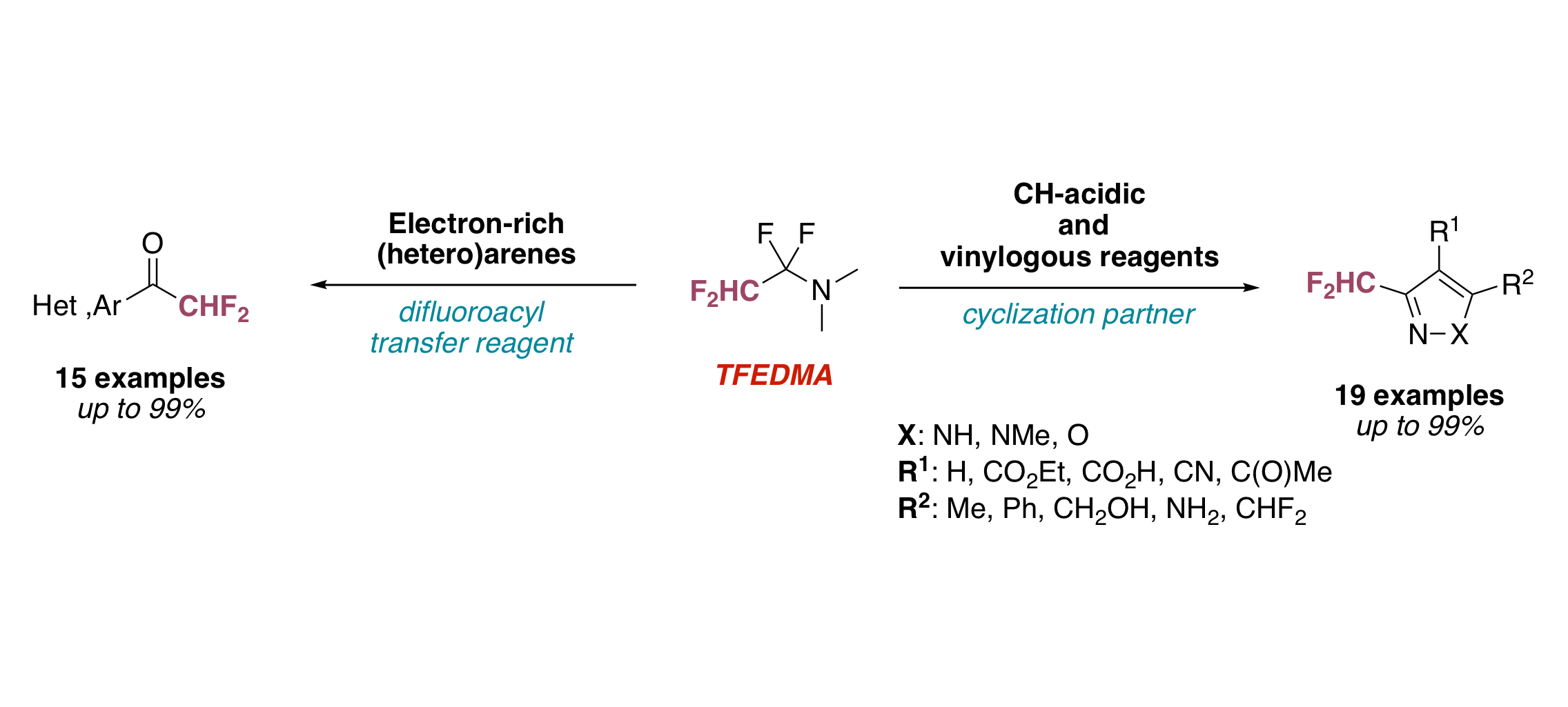
A General Approach towards NH‐Pyrazoles That Bear Diverse Fluoroalkyl Groups by Means of Fluorinated Iminium Salts
E. Schmitt, G. Landelle, J.-P. Vors, N. Lui, S. Pazenok, F. R. Leroux
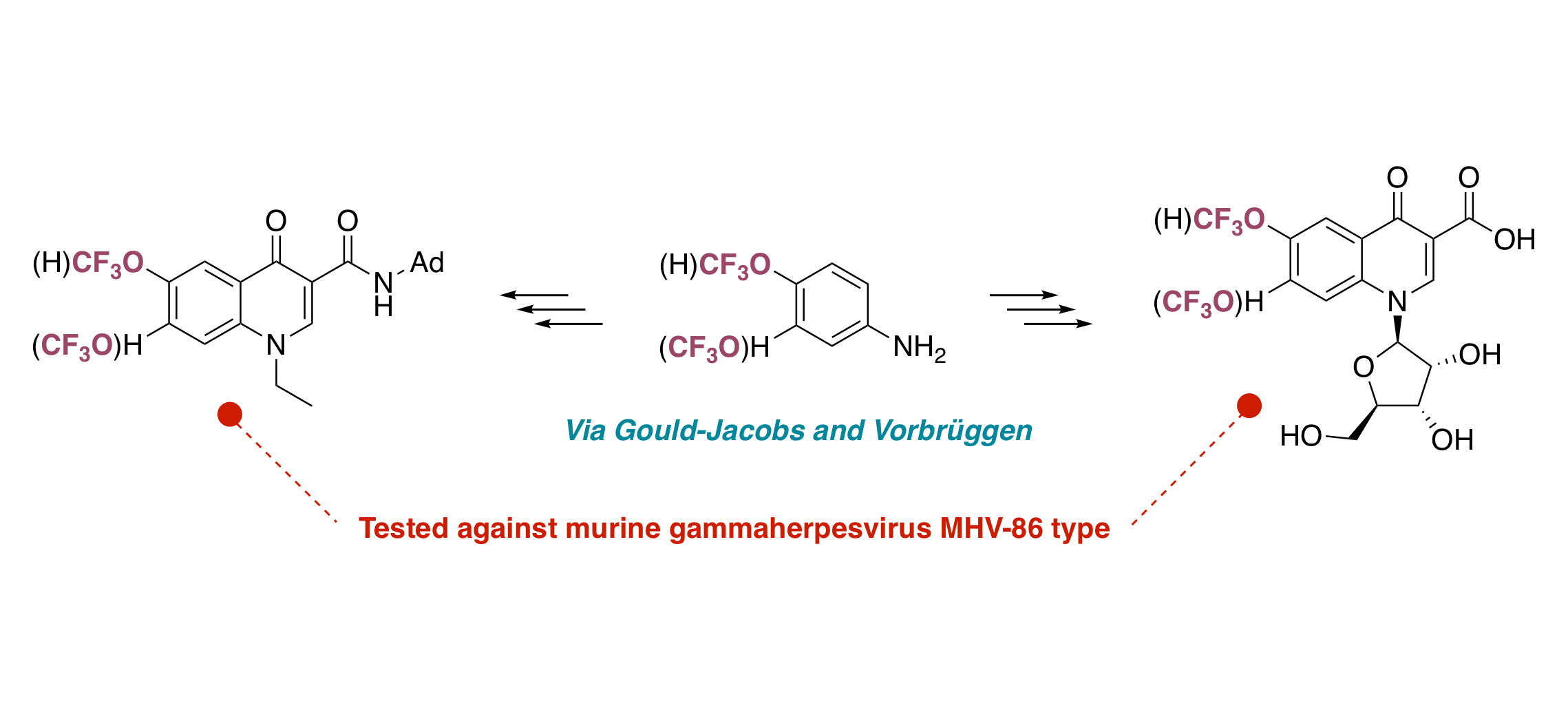
Synthesis and biological evaluation of new nucleosides derived from trifluoromethoxy-4-quinolones
K. Plevová, K. Briestenská, F. Colobert, J. Mistríková, V. Milata, F. R. Leroux
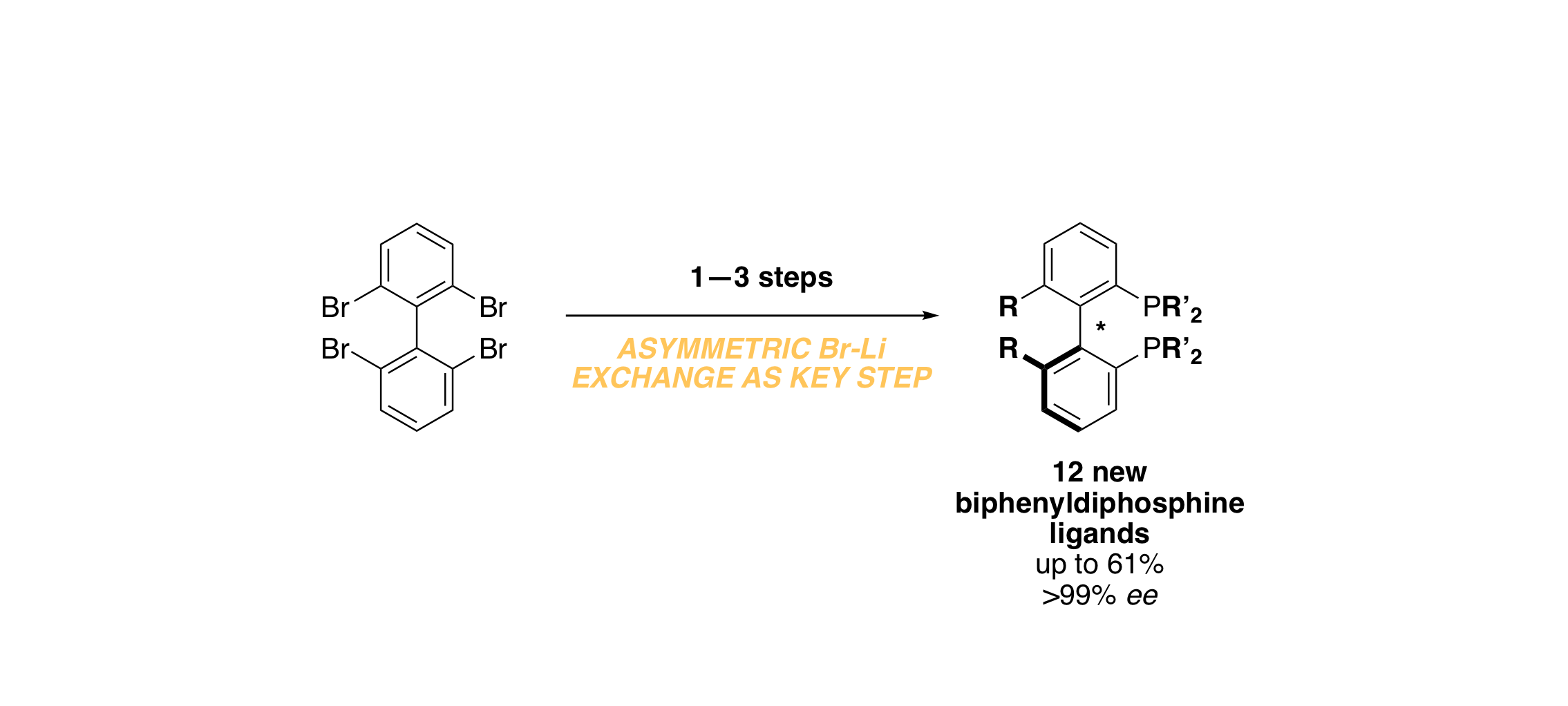
Asymmetric Bromine–Lithium Exchange: Application toward the Synthesis of New Biaryl‐Diphosphine Ligands
J. Graff, E. Łastawiecka, L. Guénée, F. Leroux, A. Alexakis
Recent advances and new concepts for the synthesis of axially stereoenriched biaryls
J. Wencel-Delord, A. Panossian, F. R. Leroux, F. Colobert
