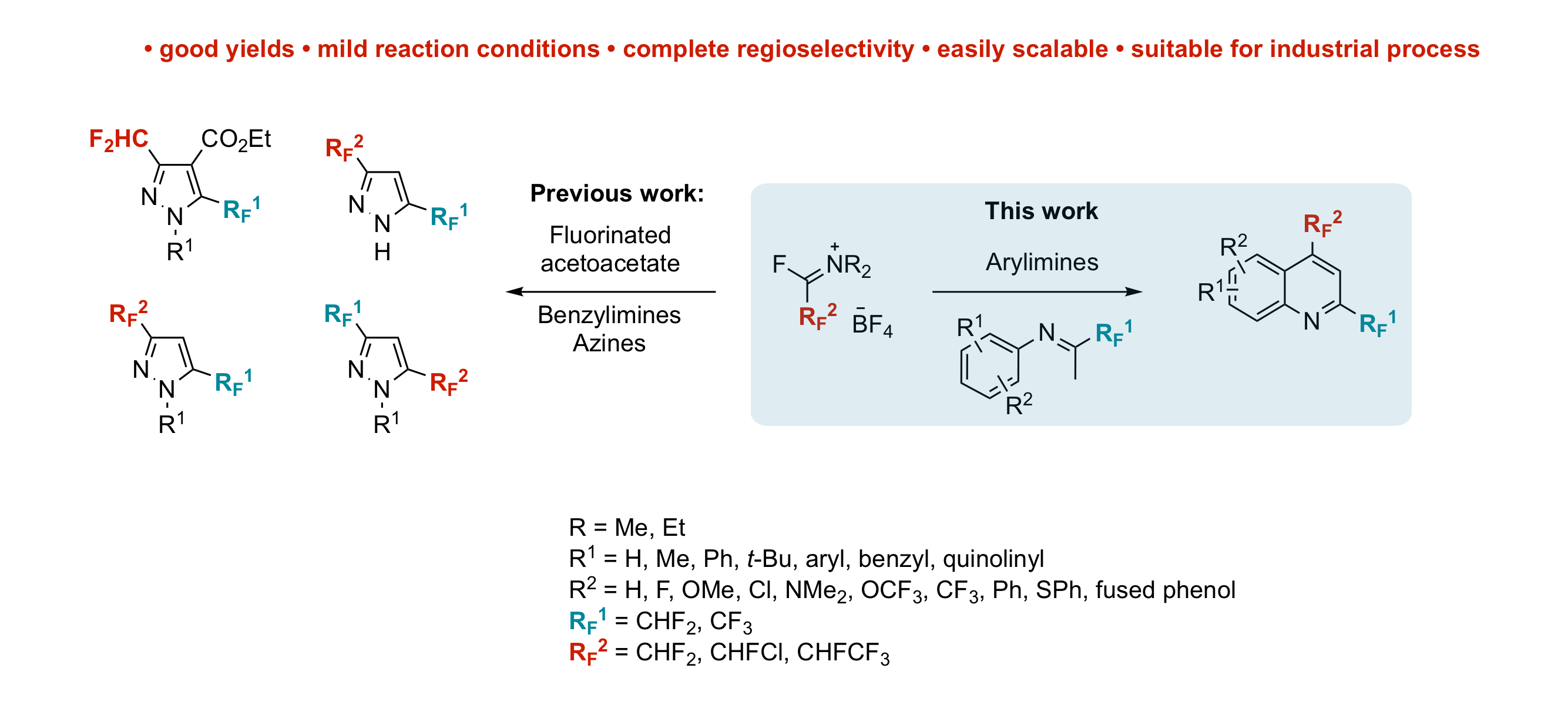
A Major Advance in the Synthesis of Fluoroalkyl Pyrazoles: Tuneable Regioselectivity and Broad Substitution Patterns
E. Schmitt, A. Panossian, J.-P. Vors, C. Funke, N. Lui, S. Pazenok, F. R. Leroux
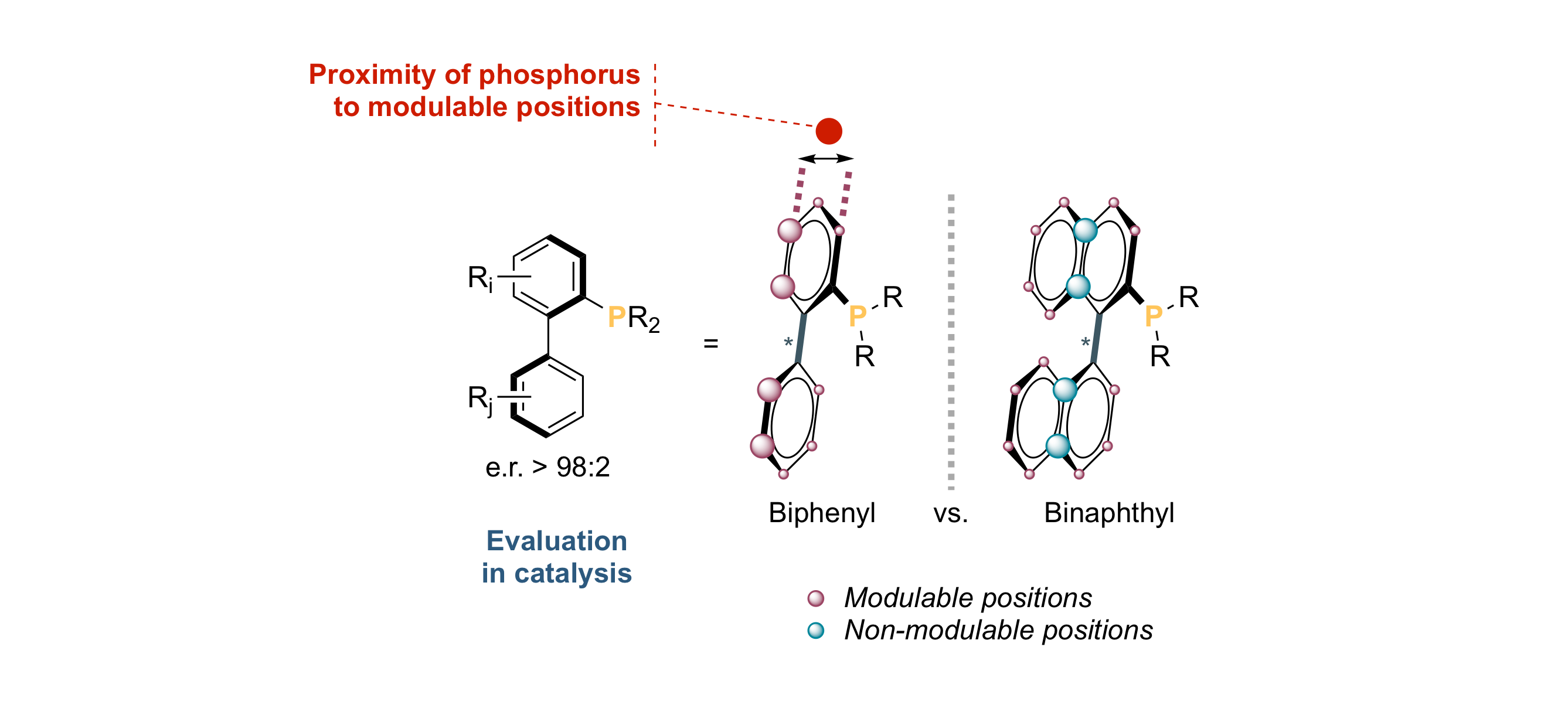
When Chirality Meets “Buchwald‐Type” Phosphines: Synthesis and Evaluation in Frustrated Lewis Pair‐, Lewis Base‐ and Palladium‐Promoted Asymmetric Catalysis
M. J. Fer, J. Cinqualbre, J. Bortoluzzi, M. Chessé, F. R. Leroux, A. Panossian
A new approach toward the synthesis of 2,4-bis(fluoroalkyl)-substituted quinoline derivatives using fluoroalkyl amino reagent chemistry
F. Aribi, E. Schmitt, A. Panossian, J.-P. Vors, S. Pazenok, F. R. Leroux
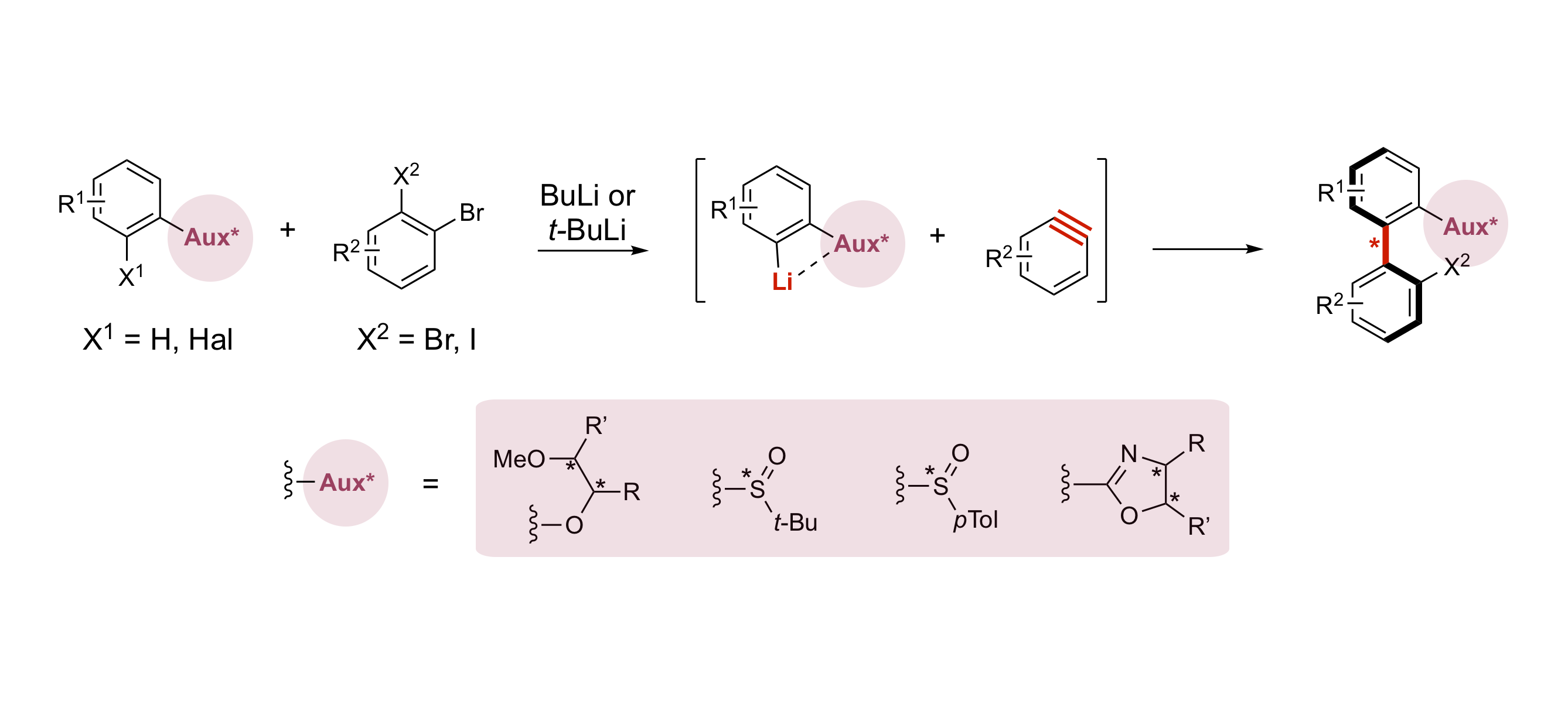
Lithium/Element Exchange as an Efficient Tool for Accessing Atropo-enriched Biaryls via Arynes
A. Panossian, F. R. Leroux
Atropo-diastereoselective coupling of aryllithiums and arynes — variations around the chiral auxiliary
D. Augros, B. Yalcouye, A. Berthelot-Bréhier, M. Chessé, S. Choppin, A. Panossian, F. R. Leroux
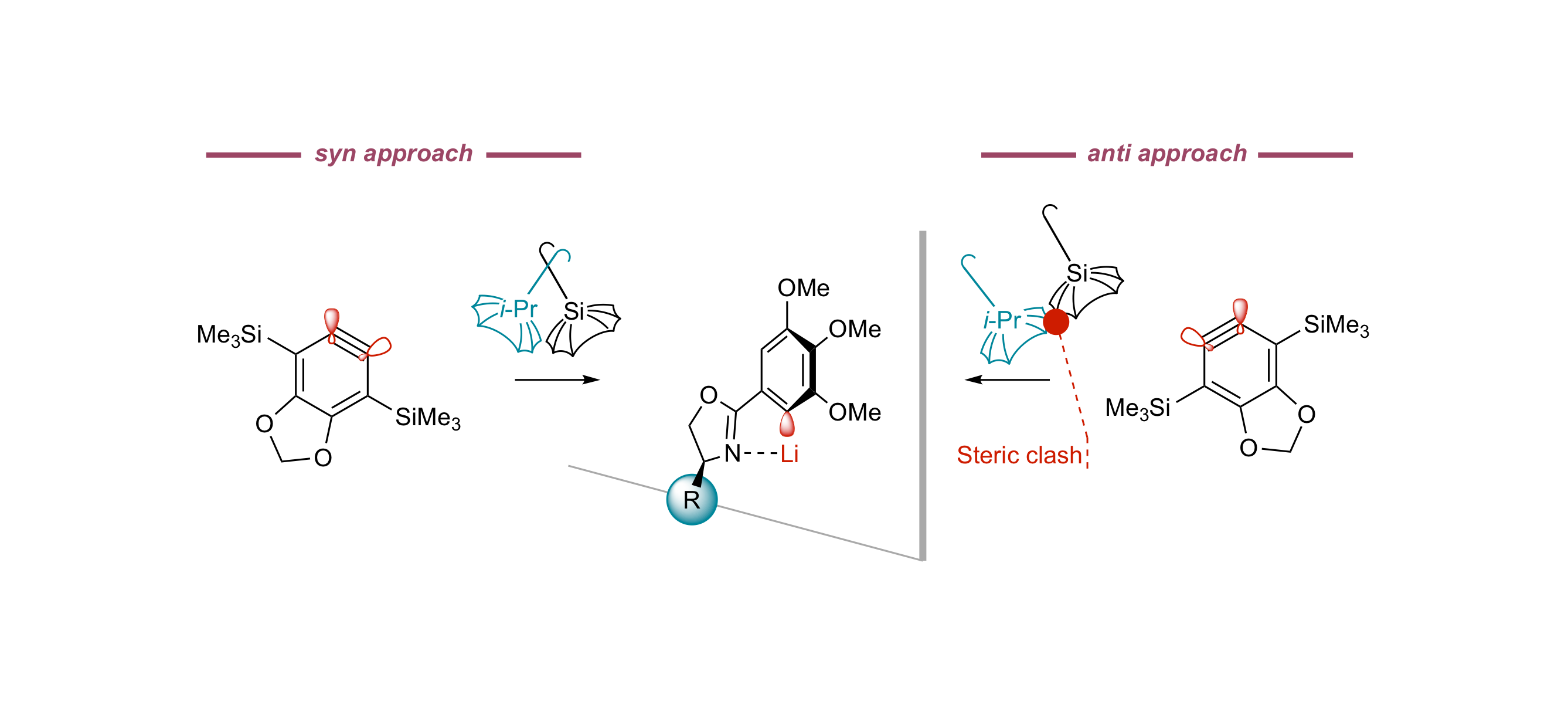
Access to Atropisomerically Enriched Biaryls by the Coupling of Aryllithiums with Arynes under Control by Homochiral Oxazolines
B. Yalcouye, A. Berthelot-Bréhier, D. Augros, A. Panossian, S. Choppin, M. Chessé, F. Colobert, F. R. Leroux